|
Product Name
|
C-Reactive Protein (CRP) Test Kit (TRFIA)
|
|
Method
|
Time Resolved Fluorescence Immunochromatographic Assay (TRFIA)
|
|
Certificates
|
ISO13495, CE
|
|
Usage
|
Vitro Diagnostic Reagent
|
|
Function
|
Inflammation Marker
|
|
Specimen
|
Whole Blood/ Serum/ Plasma
|
|
Test Kit Components
|
20 / 50 Test cards
20 / 50 Sample buffer
1 IC card 1 Instruction manual |
|
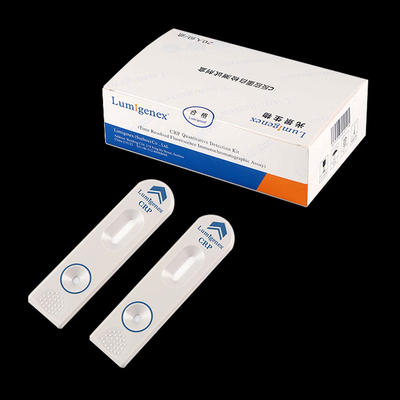
Test Card Components
|
The test card is composed of a plastic cartridge and a test strip.
The strip consists of a sample pad, a marker pad (Coated with a mouse anti-human CK-MB monoclonal antibody nano-microsphere fluorescent probe (0.5%), a Detection membrane (coated with mouse anti-human CK-MB monoclonal antibodies (1.5 mg/mL) and gout against mouse IgG polyclonal antibody (1.0 mg/mL)), absorbent paper and polystyrene (PVC) plywood.
Sample buffer, 1mL/test, mainly composed of phosphate (PBS) buffer, containing 0.5% calf serum albumin (BSA) and 0.05% preservative proclin-300 (main active ingredient Are 2-methyl-4-isothiazolin-3-one and 5-chloro-2-methyl-4-isothiazolin-3-one). |
|
Storage & Shelf Life
|
Unopened test kit is stable for 18 months under 2-8℃, and up to 30 days under 2-30℃.
Temperature change (< 37℃) during shipment has no effect on quality of the test kit. Unsealed test kit is stable within 1 hour |
|
Compatible Equipment
|
Lumigenex Time-resolved Fluorescence Immunoanalyzer LTRIC-600, LTRIC-1000
|